|
 Thyroid Science 6(2):1-7, 2011
Thyroid Science 6(2):1-7, 2011

Clinical Utility of
Soluble Fas and
Fas Ligand in Thyroid Pathogenesis

Full
Text Free
(pdf)

Dhara R.
Gajjar,1 Girish H. Patel,1 Toral P. Kobawala,1
Kamini N. Patel,1 Urvi B. Parekh,2 Deepal K.
Parekh,2
Kirti M. Patel,3 Shilin N. Shukla,4 Pankaj M. Shah5

1Division of
Molecular Endocrinology;
2Hon. Visiting
Physician & Clinical
Endocrinologist;
3Hon. Deputy Director
(Medical), 4Professor;
Medical Oncology;
5Hon. Director. The
Gujarat Cancer & Research
Institute,
NCH Compound, Asarwa,
Ahmedabad-380 016, Gujarat,
India.
Correspondence:
Dr. Girish H. Patel,
Clinical Research Associate:
Division of Molecular
Endocrinology-I,
& Incharge: Clinical
Biochemistry Laboratory,
The Gujarat Cancer & Research
Institute,
NCH Compound, Asarwa,
Ahmedabad-380 016, India
dharargajjar@gmail.com
ghpatel_961@yahoo.co.in
|
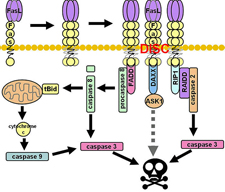
 Abstract.
Background:
The soluble form of Fas
(sFas) lacks the transmembrane
domain due to alternative splicing.
sFas blocks Fas-mediated apoptosis
by binding to Fas ligand (FasL).
This study was designed to examine
whether the apoptotic-inhibitor sFas
and the apoptotic inducer FasL are
differentially present in two
opposite phenotypes of autoimmune
thyroid disorders (AITD), nodular
goiter (NG), and thyroid cancer.
Methods:
sFas and FasL levels
were determined using ELISA in the
serum sample of a total 68 patients
with Hashimoto’s thyroiditis (HT) (n
= 15), Graves’ disease (GD) (n =
17), nodular goiter (n = 21), and
thyroid carcinoma (n = 15). These
patients’ levels and were compared
with 20 age matched, disease-free
controls. In addition, for
comparison, the levels of
thyroglobulin and thyroid peroxidase
antibodies were measured.
Results:
All studied groups had
raised sFas levels. Levels in AITD
patients were statistically
significantly higher than in
controls. FasL levels were
significantly higher in all studied
groups except the thyroid cancer
group, as compared with controls.
Compared to controls, GD patients
had higher sFas level, and the HT
group had a higher FasL level. Also,
compared to controls and the NG
group, thyroid cancer patients had
higher sFas and lower FasL levels.
Conclusion:
Fas-mediated apoptosis
plays an important role in the
active stage of the autoimmune
process of both GD and HT. Increased
sFas in GD and FasL in HT may
contribute to homeostasis in the
thyroid gland. In thyroid cancer and
NG, however, sFas and FasL may
provide a key protective signal that
helps the cells to avoid apoptosis
in a hostile environment. Abstract.
Background:
The soluble form of Fas
(sFas) lacks the transmembrane
domain due to alternative splicing.
sFas blocks Fas-mediated apoptosis
by binding to Fas ligand (FasL).
This study was designed to examine
whether the apoptotic-inhibitor sFas
and the apoptotic inducer FasL are
differentially present in two
opposite phenotypes of autoimmune
thyroid disorders (AITD), nodular
goiter (NG), and thyroid cancer.
Methods:
sFas and FasL levels
were determined using ELISA in the
serum sample of a total 68 patients
with Hashimoto’s thyroiditis (HT) (n
= 15), Graves’ disease (GD) (n =
17), nodular goiter (n = 21), and
thyroid carcinoma (n = 15). These
patients’ levels and were compared
with 20 age matched, disease-free
controls. In addition, for
comparison, the levels of
thyroglobulin and thyroid peroxidase
antibodies were measured.
Results:
All studied groups had
raised sFas levels. Levels in AITD
patients were statistically
significantly higher than in
controls. FasL levels were
significantly higher in all studied
groups except the thyroid cancer
group, as compared with controls.
Compared to controls, GD patients
had higher sFas level, and the HT
group had a higher FasL level. Also,
compared to controls and the NG
group, thyroid cancer patients had
higher sFas and lower FasL levels.
Conclusion:
Fas-mediated apoptosis
plays an important role in the
active stage of the autoimmune
process of both GD and HT. Increased
sFas in GD and FasL in HT may
contribute to homeostasis in the
thyroid gland. In thyroid cancer and
NG, however, sFas and FasL may
provide a key protective signal that
helps the cells to avoid apoptosis
in a hostile environment.
Keywords
Autoimmune thyroid
disease • Fas ligand • Graves’
disease • Hashimoto’s thyroiditis •
Nodular goiter • Soluble Fas •
Thyroid cancer
Citation:
Gajjar, D.R. Patel, G.H.,
Kobawala, T.P., Patel, K.N., Parekh,
U.B., Parekh, D.K., Patel, K.M.,
Shukla, S.N., and Shah, P.M.:
Clinical Utility of Soluble Fas and
Fas Ligand in Thyroid Pathogenesis.
Thyroid Science, 6(2):1-7,
2011.
Full
Text Free
(pdf)

© 2011
Thyroid Science |
|